
In Korea, scientists and clinicians from the Natural Products Informatics Research Center of the Korean Institute of Science and Technology (KIST) have managed to develop an innovative mathematical model for pancreatic cancerwhich includes acquired resistance and plasticity of cancer cells.
In the study, which appears in Chaos, Solitons & Fractalsits authors explain the development of the mathematical model of tumor dynamics with acquired resistance.
Recent preclinical and clinical work suggests that evolution-based cancer therapies that exploit intratumoral competition can significantly delay resistance.
Various mathematical and computer models have been used to explain the effectiveness of particular treatment strategies. However, this evolutionary approach often assumes only pre-existing resistance, although Acquired resistance and plasticity of cancer phenotype can dramatically affect treatment outcomes.
As these scientists highlight in their work, they theoretically investigated whether an evolutionary therapy that takes advantage of plasticity could delay resistance. “In particular,” they say, “we propose a time-dependent evolutionary dose that can modulate the evolving competition between plastic and drug-sensitive cells. We assume a type competition model Lotka-Volterra of cancer cell populations, with constant loading capacity, to analyze the dynamics of tumor growth and show that some tumors can be controlled with the right dosewhich balances the competition between different cell species to coexist in a stable tumor below a tolerable load.”
Adaptive therapy
In this sense, they argue that “the appearance of a new resistant species can further increase the volume of the tumor, which can be canceled by reducing the dose. In contrast, increased tumor volume due to new metastatic formation requires a higher dose. Furthermore, we propose adaptive programming based on tumor dynamics that consists of subsequent treatment holidays and periods of low and high doses. “This approach can contain the tumor at variable volumes below the tolerable load, which can facilitate reiteration of the treatment strategy in case of any evolutionary change.”
In general -they clarify-, “this study provides a theoretical understanding of the evolutionary dosage that may delay resistance”.
It is well known that cancer poses significant challenges due to the development of resistance and the likelihood of relapse. Resistance can arise from permanent genetic changes in cancer cells or from non-genetic alterations in cancer cell behavior induced by treatment.
The standard of care in cancer treatments generally involves administering the maximum tolerated dose of a drug to effectively eradicate drug-sensitive cells.
However, this approach often fails in the long term, because Resistant cancer cells can grow more quickly when all drug-sensitive cancer cells are killed.
An evolution-based treatment approach, called adaptive therapy, personalizes the dose or treatment breaks based on the patient’s individual responses. Its goal is to maintain a sufficient number of sensitive cells to control the growth of resistant ones. Recent studies and clinical trials have shown that the adaptive therapy could delay resistance more effectively compared to standard treatment.
Mathematical model
Determining dosage and treatment breaks for each patient is challenging because cancer is a complex, evolving system and every patient is different. Mathematical models can be useful for designing strategies.
In fact, several mathematical models have been developed to explore the effects of various treatment strategies on patient outcomes. However, existing models often overlook the impact of acquired resistance and plasticity of cancer cells.
The acquired resistance It encompasses several types of resistance that often arise due to genetic changes. The cellular plasticity refers to the ability of cancer cells to alter their phenotypes in response to changes in their microenvironment, such as fluctuations in treatment dosage or cessation.
This study, led by Dr. Kim Eunjung, has established a theoretical basis for cancer treatment strategies following tumor evolution.
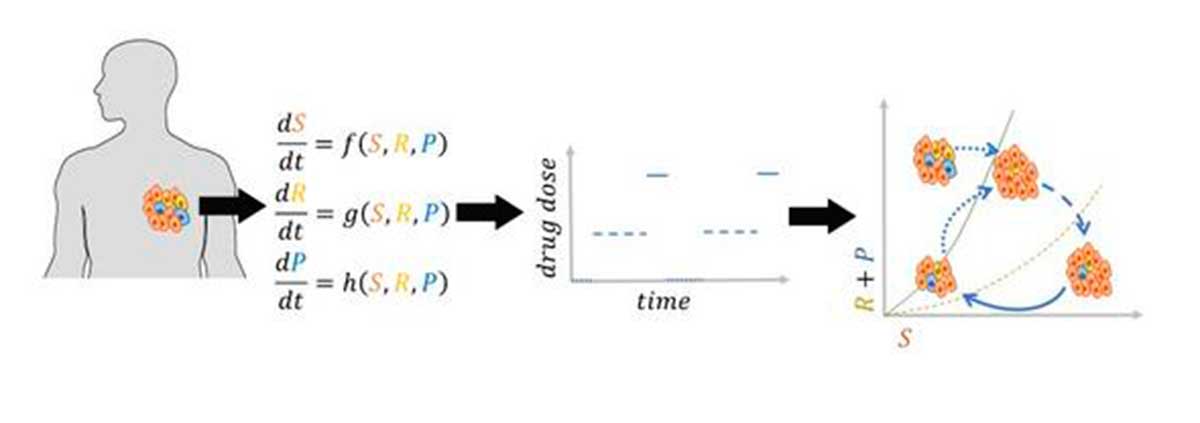
They have developed a mathematical model to predict the evolution of tumorsconsidering the acquisition of resistance by cancer cells and their ability to alter phenotypic behavior (plasticity) during treatment.
The analysis of their model has identified the conditions for the existence of an effective dose window, a range of doses that could keep the tumor volume at an equilibrium point, unchanged and stable.
For some tumors with plasticity, taking breaks in treatment helps cancer cells become sensitive againjoining forces with other sensitive cells to suppress the growth of resistant cells.
Mathematical model and effective dose range
These researchers have proposed a dosage of evolutionary therapy which involves administering the treatment in cycles that include treatment holidays, minimum effective doses and maximum tolerated doses.
Pausing treatment allows plastic cancer cells to regain sensitivity, followed by application of a minimally effective dose to control tumor volume. Subsequently, a maximum tolerated dose is administered to further reduce the size of the tumor.
This dosing cycle effectively contains the tumor volume to a manageable level. Numerical simulations of the proposed strategies, applied to a patient with melanoma, further illustrate these findings. The results show that evolutionary dosing can redirect tumor dynamics, keeping its size below a tolerable burden.
Always according to these researchers, The mathematical model they have developed can predict the effective dose range of drug candidates for cancer treatment before clinical trials. Additionally, it can help determine the anticancer effects of new treatments and identify the effective dose range for each drug.
Ultimately, the model contributes to personalized cancer treatment strategies by considering patient-specific tumor evolutionary dynamics during treatment.
In relation to the work carried out, Dr. Eunjung explains that “in the current study, we emphasize the role of phenotypic plasticity of cancer cells in improving the controllability of tumor burden with cyclical doses of evolutionary treatment.”