In the dynamic healthcare innovation landscape, Montreal-based startup FemTherapeutics is proving to be a key player in redefining women’s health through personalized solutions. FemTherapeutics specializes in the treatment of pelvic organ prolapse (POP) and is a pioneer in the development of 3D printed pessaries that are carefully tailored to each patient’s individual needs. We had the privilege of speaking with them to learn about the company’s history, its methods and its future plans. Vince, Director of Mechanical Engineering R&D, told us about the origins of FemTherapeutics, their use of medical 3D printing and the potential of their innovative approach.
3DN: Could you briefly introduce yourselves? How were your beginnings in 3D printing?
Vincent Castonguay-Siu, Director of Mechanical Engineering R&D at FemTherapeutics
We are a Montreal-based startup focused on revolutionizing women’s health through personalized treatment for pelvic organ prolapse (POP). To do this, we develop 3D printed pessaries that adapt to each person. The custom look of these devices is based on anatomical and pathological data that is fed into our models to optimize the model geometry for each patient. I’m Vince, Director of Mechanical Engineering R&D at FemTherapeutics. I have a master’s degree in Biomedical Engineering from ETH Zurich and 7 years of experience in medical device design and manufacturing. I have received several awards for design and innovation, nationally and internationally.
I was introduced to 3D printing in high school and university. I used the technology to prototype scoliosis corsets, robotic rehabilitation gloves, and disc implants. But I really became a maker when I joined the ETH Makerspace as a volunteer manager, and I haven’t stopped building since. Inara is co-founder and CEO of the company. Before venturing into FemTherapeutics, Inara led Fortune 500 companies through major digital transformations and product launches in her role as a technology consultant at E&Y. Negin Ashouri is Co-Founder and CTO of FemTherapeutics. She has four years of experience in robotics and has won several international awards in these fields. Dr. Mihnea Gangal is Co-Founder and CMO of FemTherapeutics. He is a practicing urogynecologist at Notre Dame and St Mary’s hospitals in Montreal.
3DN: How did FemTherapeutics come about? Why do you use 3D printing?
FemTherpaeutics is a spin-off project of McGill’s Surgical Innovation Program. This program is designed to bring together students from multidisciplinary backgrounds and give them the opportunity to immerse themselves in the hospital environment to understand the challenges faced by both surgeons and patients. In the gynecology department, Negin and Inara spent many hours in the operating rooms accompanying Dr. Gangal during his interventions. They realized that many women needed surgery for a common problem, pelvic organ prolapse (POP). After consulting with their supervisor at the time, Negin and Inara discovered the shortcomings of current non-invasive solutions for POP, which were leading to an increasing reliance on surgery. Therefore, they decided to take a step forward in solving this problem by developing a dynamic and personalized pessary that would improve the quality of life of patients and provide them with comfort. As a result, Dr. Gangal became one of his co-founders in this endeavour.
Negin (left), Mihnea (center) and Inara (right).
At FemTherapeutics we conclude that 3D printing is the most suitable technology for the development of personalized medical devices. It is one of the few technologies capable of producing personalized geometries that meet the quality standards we demand, with the profitability necessary to develop a viable product.
3DN: What 3D printing process and materials do you use?
We generally use stereolithography (SLA) 3D printing technology for the development of pessaries. The main reason is the platform’s ability to print the complex geometries required for the treatment of pelvic floor disorders without the need for excessive manual post-processing. Furthermore, the high resolution and smooth surface achieved with this technology facilitates cleaning and minimizes the risk of bacterial proliferation. Depending on the application, we work with different materials. For the development of pessaries, we considered medical silicone or silicone-like materials from Formlabs, Loctite and Carbon. For the development of functional structural prototypes, molds and devices, we like to work with technical photopolymer resins (Elastic 50A, Flex 80A, Tough 2000) and filaments (PLA, PETG, TPEs). We select our materials based on the functional requirements of the design (stiffness, hardness, surface finish, etc.).
3DN: How does gynecological prosthesis work for the treatment of POP and urinary incontinence?
One in four women suffers from pelvic floor disorders, such as incontinence and pelvic organ prolapse (POP). Current treatment options include pelvic floor surgery and vaginal pessaries. 20% of North American women undergo pelvic floor surgery, which has a 30% failure rate and a high risk of complications. Pessaries are intravaginal support devices that are mass-produced in geometric shapes and sizes. More than half of pessary users stop using them within the first year due to complications.
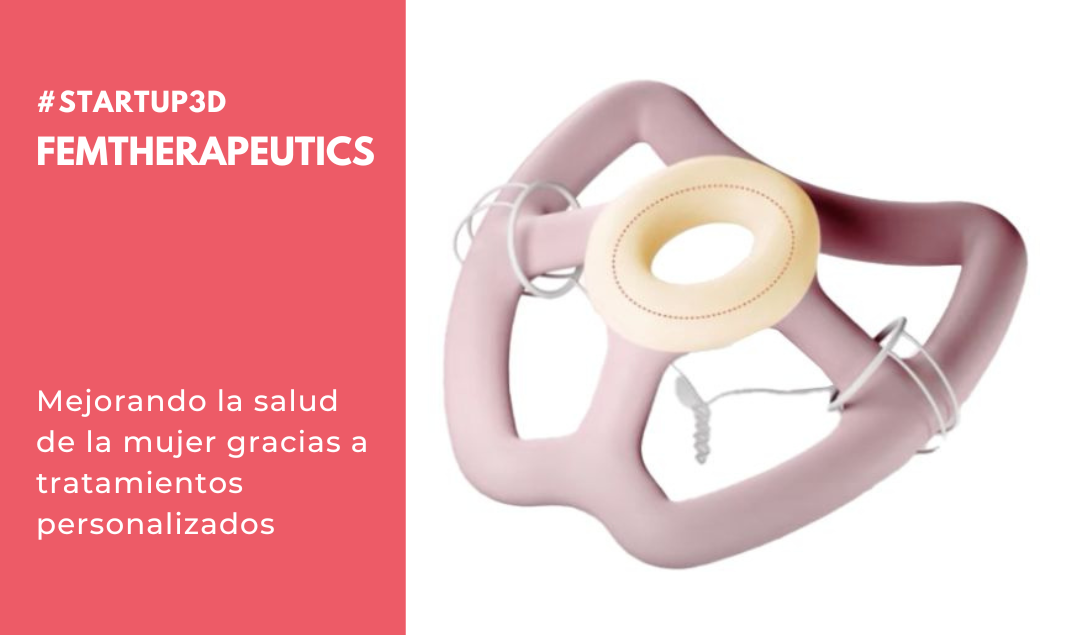
FemTherapeutics is developing the world’s first customizable gynecological prosthesis (or vaginal pessary) that provides effective symptomatic relief of pelvic floor disorders. Thanks to the latest advances in cloud software and artificial intelligence, we have developed a comprehensive data-driven pelvic health platform. This allows doctors to visualize each patient’s condition in 3D software and then suggest pessary designs tailored to the patient’s unique anatomical characteristics. Once the designs are finalized, we use state-of-the-art 3D printing techniques to manufacture directly from medical grade silicone that has never been used in gynecology before.
3DN: What are the next steps for FemTherapeutics and your long-term goals?
At FemTherapeutics we are currently working on freezing the pessary design. We will then complete biocompatibility testing to obtain approval to conduct a pilot clinical trial for regulatory approval. The design of the measuring device has been frozen and we are currently seeking a manufacturer to obtain samples for regulatory approval and distribution, which is planned for the third quarter of 2024. FemTherapeutics’ vision is to become the most personalized solution effective for women’s health. After completing our first product, we intend to expand our technology to other areas such as maternal health, oncology and menstruation.
3DN: Any last words for our readers?
We understand that personalized medicine and decentralized technological platforms such as 3D printing are going hand in hand. TO As the latter evolves, personalized medicine will also become ubiquitous. For more information about FemTherapeuticsvisitto their website, HERE.
The FemTherapeutics Team
What do you think of the activity carried out by FemTherapeutics? Leave your comments on our social networks: Facebook, Twitter and YouTube. Follow all the information about 3D printing in our weekly Newsletter.
*All photo credits: FemTherapeutics