by Linda Wang
Although people with advanced ovarian cancer can initially be treated with platinum-based chemotherapy drugs, the cancer often comes back. Platinum-resistant ovarian cancer is difficult to treat. However, the Food and Drug Administration (FDA) recently granted final approval for the use of mirvetuximab soravtansine-gynx (Elahere), which offers a new treatment option for some of these people.
Under approval, Elahere is used to treat people with platinum-resistant advanced ovarian cancer whose tumors overproduce a protein called FR-α. About 80% of people with high-grade serous epithelial ovarian cancer, the most common form of the disease, have tumors that overexpress FR-α.
The FDA approval was based on the results of a large randomized clinical trial called MIRASOL. The results indicated that people with FR-α-positive platinum-resistant ovarian tumors who were treated with Elahere lived longer overall than those who received standard chemotherapy. This drug also prolonged the time they lived without the cancer getting worse, known as progression-free survival. Participants treated with Elahere also had fewer serious side effects.
The approval of Elahere is a long-awaited milestone in the treatment of advanced ovarian cancers, noted MIRASOL study principal investigator Dr. Kathleen N. Moore of the University of Oklahoma Health Sciences Center.
For people whose disease becomes resistant to platinum chemotherapy drugs, “this is the first new therapy… that improves overall survival,” Dr. Moore said, adding that “it is also the first drug in a decade that improves progression-free survival.
With the availability of this new treatment, it is essential to test tumors for FR-α, Dr. Moore added. “We need to know if there is FR-α in the patients’ tumors to use this drug as soon as possible when the tumors become resistant to [la quimioterapia con] platinum”.
Target a protein that is only in cancer cells
Ovarian cancer is the fifth most common cause of cancer death in women in the United States. In 2023, there were an estimated 19,710 new cases and 13,270 deaths from the disease. Most people are not diagnosed until the advanced stage because the signs or symptoms of early disease are not specific.
Treatment of advanced disease usually includes surgery followed by platinum-based chemotherapy. But the disease will return in about 80% of people, often because it becomes resistant to chemotherapy.
A standard treatment for these patients, a combination of targeted therapy with bevacizumab (Avastin) and chemotherapy (non-platinum), was shown to produce a modest increase in progression-free survival in people with platinum-resistant ovarian cancer. platinum, but not overall survival.
Researchers have tried to help people whose cancer becomes resistant to platinum chemotherapy by creating drugs that target FR-α. Overexpression of FR-α in many ovarian cancers is not only common, but about 30% to 40% of high-grade serous ovarian cancers produce it at very high concentrations.
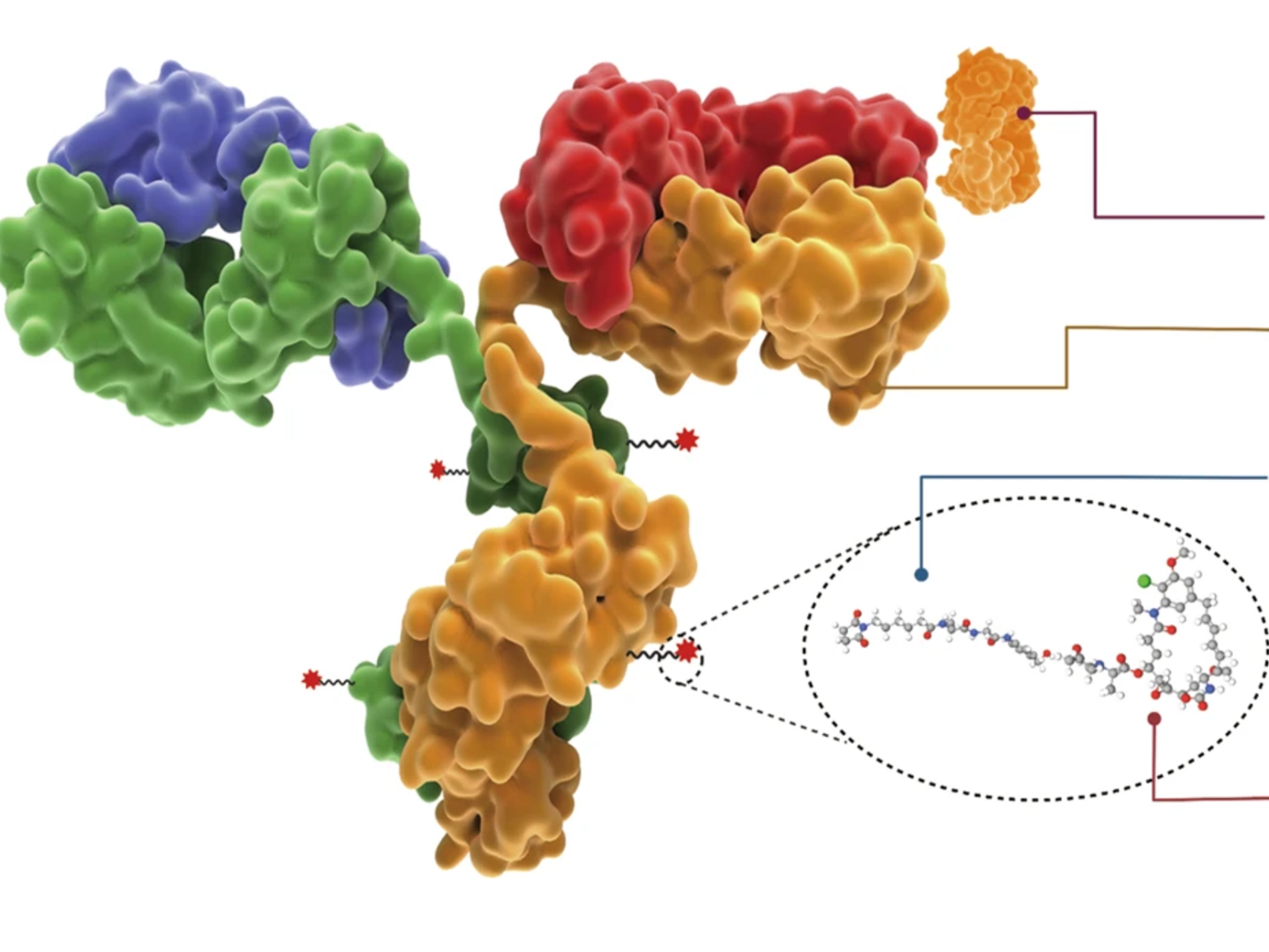
Elahere is an antibody-drug conjugate composed of a monoclonal antibody, called mirvetuximab, linked to a very potent chemotherapy drug called DM4. After patients receive it via infusion, mirvetuximab seeks out and binds to FR-α on the surface of ovarian cancer cells. The drug then enters the cancer cell where DM4 is released and destroys the cell. Since FR-α is mostly found in cancer cells, it does not usually damage healthy cells.
In a large clinical study of patients with platinum-resistant, FR-α-positive ovarian cancer, the use of Elahere showed great promise in treating tumors with the highest concentrations of the FR-α protein. In a later study called SORAYA, limited to those with high levels of FR-α, treatment with Elahere shrank tumors in almost 40% of people.
Based on these results, the FDA granted accelerated approval for the use of Elahere in November 2022. The FDA requested that the MIRASOL study confirm that the drug offered clinical benefits, including improved survival.
Help prolong patients’ lives
Immunogen, the drug’s manufacturer, funded the MIRASOL study. In this, 453 people with platinum-resistant, FR-α-positive ovarian cancer were randomly assigned to receive Elahere as an intravenous infusion every 3 weeks or to chemotherapy.
Treatment with Elahere not only increased overall survival and progression-free survival, but it made tumors almost three times more likely to shrink compared to those who received chemotherapy. In some people who received the drug, the cancer completely disappeared for a time.
| Survival without progression | Overall survival | Patients with reduced tumor volume | |
| Elahere | 5.6 months | 16.5 months | 42% |
| Chemotherapy | 4.0 months | 12.8 months | 16% |
People treated with Elahere had fewer serious side effects, such as low blood counts, than people treated with chemotherapy. Eye problems, such as blurred vision and corneal conditions (keratopathies), were some of the most common side effects of Elahere.
Elahere includes a special warning about eye-related side effects. According to Dr. Moore, side effects are usually treated with corticosteroid eye drops or a lower, more frequent dose of treatment is used.
“We have not had patients with permanent eye damage. And once it resolves, it usually doesn’t happen again, so patients can continue taking the medication,” Dr. Moore said.
A specialist in the treatment of gynecologic cancers, Dr. Caroline Billingsley of the University of Cincinnati College of Medicine, said it is important for oncologists who prescribe Elahere to work closely with optometrists and ophthalmologists to manage any symptoms that occur. arises related to the eyes.
Dr. Billingsley, who was not involved in the study, said the patients she treated with Elahere generally have a good quality of life.
“They are not having the side effects that usually appear with standard chemotherapy,” he said.
“This medication allows them to avoid alopecia (hair loss), which is great. It’s also a little better for neuropathy (nerve pain).”
A new era for ovarian cancer treatment
Elahere is currently being evaluated in people with FR-α-positive, platinum-sensitive ovarian cancer that has returned after treatment. Researchers are also studying whether Elahere is effective as an initial or first-line treatment for advanced ovarian cancer and in combination with bevacizumab.
According to Dr. Moore, “it will take several years to figure out” the best way to use Elahere to treat ovarian cancer.
He added that there are other antibody-drug conjugates being studied to treat ovarian cancer that also look promising, most with targets (molecules where the therapy is directed) other than FR-α.
Regarding the recent approval, he noted that this “marks the beginning of a new era.” “I think, in a few years, we will have an even better medicine.”