by Nadia Jaber
Based on the results of a large study, scientists created a blood test that accurately detects early-stage pancreatic cancer. This is a liquid biopsy, a test that uses blood or other body fluids to detect or monitor cancer.
In the vast majority of people with pancreatic cancer, this cancer is detected when it has spread to other organs and is not operable. This is because early-stage disease does not cause obvious symptoms and there are no reliable tests to detect it.
But if pancreatic cancer is found earlier, the chance of surviving 5 years after diagnosis is much higher: 44% for early-stage disease versus 3% for advanced-stage disease.
The blood test, created by Dr. Ajay Goel of the Duarte Cancer Center at City of Hope in California, and his colleagues, analyzes small fragments of ribonucleic acid (RNA) released by tumors. In the new study, which included nearly 1,000 people from several countries, the test accurately detected early-stage and advanced-stage pancreatic cancer in a large, diverse group of people.
And when researchers combined use of the blood test with another test that detects a protein called CA19-9, it accurately identified 97% of people with early-stage pancreatic cancer. High accuracy means that a test is not only effective at detecting those who have cancer, but is also effective at not detecting it in those who do not have cancer.
Dr. Goel presented the results at the American Association for Cancer Research (AACR) annual conference on April 8. However, the results have not yet gone through external scientific review, a process in which other scientific experts in the field evaluate scientific quality and accuracy.
“Pancreatic cancer is devastating,” said Dr. Matthew Young, program co-director of the Pancreatic Cancer Detection Consortium (PCDC) at the National Cancer Institute (NCI), which funded the study. “Once the patient receives the diagnosis [de enfermedad en estadio avanzado], they usually have 3 to 6 months to live. So anything we can do to change this is important.”
But in the general population, where the risk of pancreatic cancer is low, even a test with near-perfect accuracy would give many false-positive results, Dr. Young explained. If a test indicates that someone might have pancreatic cancer, abdominal surgery is needed to prove it, he said.
However, for people at higher risk for pancreatic cancer, the benefits are more likely to outweigh the harms of screening, he added.
“The most effective and practical use of [esta] blood test would be in high-risk people,” agreed Dr. Goel. This includes people with an inherited genetic mutation that puts them at high risk for pancreatic cancer, people with family members who have had pancreatic cancer, and people with chronic pancreatitis or new-onset diabetes.
But the test must be studied further before it can be used to screen people for pancreatic cancer, said Dr. Howard Crawford, scientific director of the Henry Ford Pancreatic Cancer Center in Michigan, who was not involved in the study.
Using microRNAs to detect cancer
For more than a decade, researchers studied whether it was possible to use microRNAs (a type of RNA) to detect cancer.
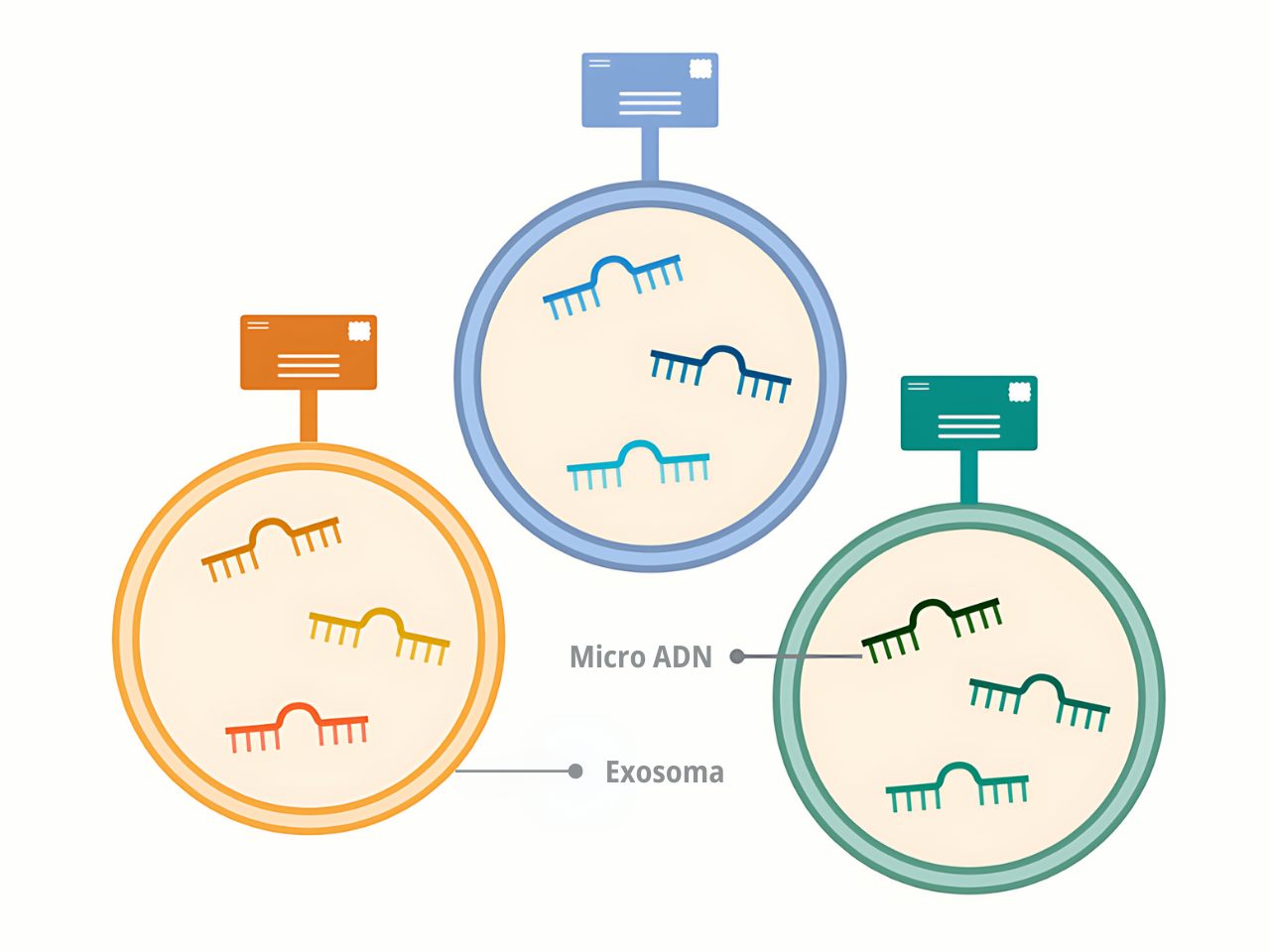
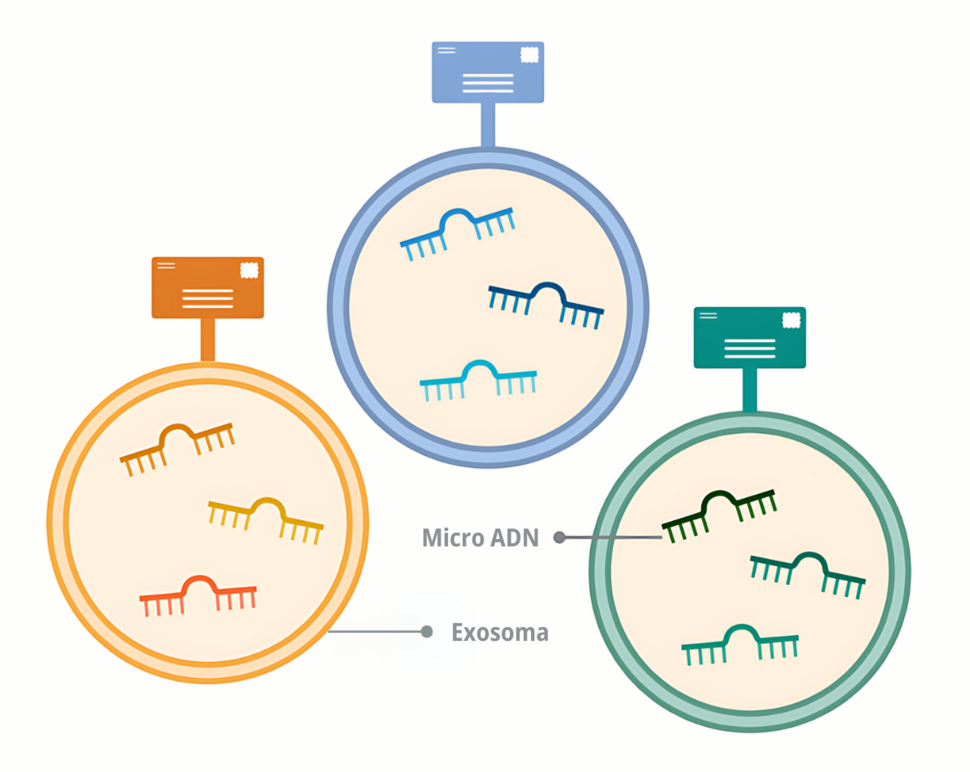
Dr. Goel indicated that microRNAs are the ideal molecules to detect cancer because they are abundant and stable in the blood, and tumors release them in enormous quantities. Some of these microRNAs circulate freely, while others are packaged in small bags called exosomes.
Dr. Young explained that it is easy to measure the concentrations of free microRNAs in people with cancer, but it is difficult to know where those microRNAs (or the cancer) come from.
On the other hand, microRNAs packaged in exosomes offer something that free microRNAs do not: a kind of zip code. Each organ in the body releases exosomes with a unique mark, Dr. Goel explained.
By analyzing these marks, it is possible to know if the exosomes in the blood come from the pancreas, he clarified.
In an initial study, Dr. Goel and his team found that analyzing free microRNAs and microRNAs packaged in exosomes was the key to detecting early-stage pancreatic cancer. In more than 100 people already diagnosed with the disease, the test accurately detected early-stage and advanced-stage pancreatic cancer.
“First of all, [los microARN libres] indicate “yes, we detected cancer.” Second, exosomes indicate “the cancer is in the pancreas,” Dr. Young explained.
Following promising results from the initial study, researchers prepared to test the concept in a larger, more diverse group of people.
Validation of a liquid biopsy to detect pancreatic cancer
For the current study, the team obtained blood samples from people in Japan, the United States, South Korea and China. About 500 of the participants had confirmed pancreatic cancer (including early-stage and advanced-stage disease); the other participants were considered healthy controls.
In the Japanese group or cohort, microRNA testing differentiated between people with pancreatic cancer and those without it. The test was then validated in the other three cohorts and the results were 93% accurate in the American cohort, 91% in the Korean cohort, and 88% in the Chinese cohort.
When detecting early-stage pancreatic cancer in the US cohort, microRNA testing alone was 91% accurate and 97% accurate in combination with a CA19-9 test. In the researchers’ initial study, the accuracy of CA19-9 testing alone was 86% for early-stage pancreatic cancer.
Follow-up studies to resolve important questions
Researchers are currently using the microRNA test to analyze blood samples collected in the NCI Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Study.
Because those samples were obtained years before some participants received a cancer diagnosis, the follow-up study will confirm whether the test is able to detect pancreatic cancer earlier than traditional diagnostic tests such as CT scans, Dr. Goel said. .
Dr. Crawford pointed out that the accuracy of the tests also needs to be checked in people with certain diseases.
He explained that, “pancreatic cancer and chronic pancreatitis have many cellular and molecular characteristics in common, which is why it is essential to include patients with chronic pancreatitis in follow-up studies.”
“Many promising early detection techniques fail because they fail to distinguish between pancreatic cancer and non-cancerous pancreatic disease, particularly chronic pancreatitis,” he added.
In the future, people with cancers “that share common mutations with pancreatic cancer, such as lung cancer and colorectal cancer,” should also be included in studies, Dr. Crawford said. That would ensure that the test picks up signals specific to pancreatic cancer that are not from any other type of cancer, he explained.
And finally, the only way to know if a cancer screening test truly benefits patients is to know if it reduces cancer deaths in a randomized clinical trial.