A bubble of absolute silence has sheltered the boy Aissam Dam throughout his life. Eleven years in the shadows of a blunt silence. Not a word, not a scream, not a voice had ever crossed the walls of that rare congenital deafness that has marked his life since he was born. In his native Morocco, he never learned to write or read, he barely went to school and he communicated with his family through gestures, in an unregulated sign language, just to make himself understood. This is how he lived until a few twists and turns of life, a novel gene therapy and several thousand kilometers in between took him, hand in hand with the Sant Joan de Déu Hospital in Barcelona, to a children’s health center in Philadelphia, in the United States. where science illuminated the world of sounds for the first time. His mother, Naima Tbir, emotionally remembers that day, just seven months ago: “The first day after the surgery, the father made a video call with him and the boy told me [con signos]: ‘I’m hearing the sounds of the phone!’
Aissam has become the first person in the Western world (Europe and the United States) to receive gene therapy to treat congenital deafness. And her case, furthermore, has been a success: she has recovered her hearing and identifies sounds, although her doctors warn that, due to his age, she is already late to learn language and will probably never be able to speak with fluency.
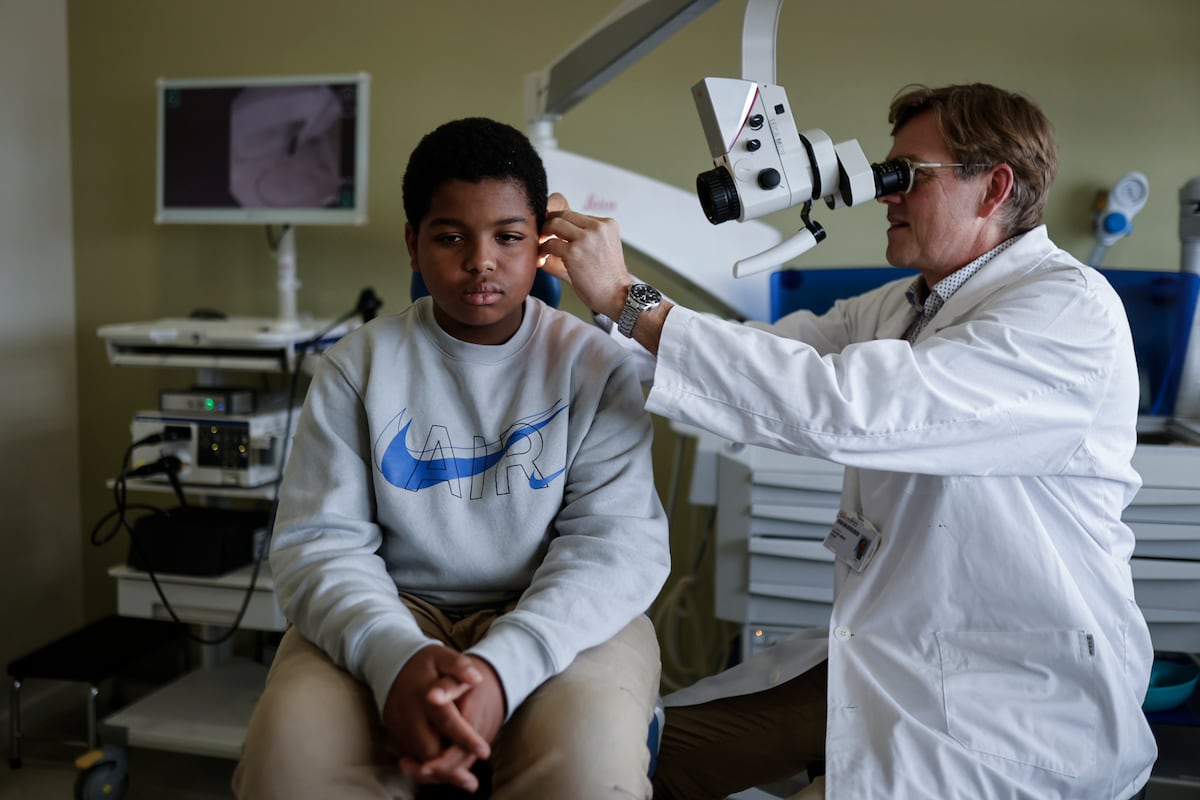
Through the colored corridors of Sant Joan de Déu, Aissam drags his feet with his head down. He is angry. He doesn’t want photos, questions, or medical check-ups. He sulks while his doctor, Oliver Haag, head of ENT at the hospital, inspects his right ear, but he’s not much up for stories. “He is angry because he wants to go to school, he doesn’t want to miss a single moment of class,” explains his mother through a translator who accompanies her. The world of sounds that has opened up to the boy, who has already turned 12, has given him a new life and even school looks different: “Aissam was born deaf. We knew this because we have two older children who also do not listen or speak. He went to school in Morocco for a year, but he didn’t understand anything,” laments Naima.
The boy suffered from a very rare disease, deafness caused by a mutation in the otof gene, key to hearing. “It is an autosomal recessive inheritance mutation, which is why there are families with several affected children. Parents can be healthy carriers, but if their children get the mutation from both parents, they suffer from the disease,” explains Haag. Only 20,000 people in Europe suffer from it: “The otof gene encodes otoferlin, a small protein in the cells within the cochlea that transmit sound in the fluid of the cochlea,” explains Haag. In a healthy ear, sound travels like air waves until it reaches the kind of snail shell that is the cochlea. There, the waves are transmitted through the liquid that bathes the interior of this conch to the auditory nerve: specifically, in this cochlear sea, there are sensory cells with hairs (ciliated cells) that function as sensors of the vibrations caused by the conch. sound and are responsible for transmitting the sound stimulus to the brain. “Otoferlin is one of those proteins that, if it does not work well, prevents the hair cell from transmitting the stimulus correctly. Nerve stimulation does not work. The chain is interrupted and the information does not reach the brain correctly. But if that gene, that protein, is repaired, the entire chain works again.”
Aissam and his family moved to Barcelona in 2018. The father, who had migrated to Spain 19 years ago, managed to complete a family reunification process and the little boy, along with his mother and siblings, joined him in the capital. Catalan. He returned to school and there, “thanks to the school staff and a Moroccan friend with a son with a similar problem,” says Naima, they entered the Sant Joan de Déu care circuit to treat his deafness.
A genetic study confirmed this mutation in the otof gene and, while the little boy continued with his visits and check-ups at the hospital, Haag’s team embarked on an international study to scrutinize the natural history of this same disease. That research was the seed of the gene therapy that was later developed and Aissam turned out to be a particularly paradigmatic case that suited the entire scientific revolution that was taking place like a glove.
“Aissam was a particular case because he has this mutation, he is deaf and he does not have a cochlear implant,” explains Haag. It is a rare profile in Western countries, where this type of pathology is usually treated in early childhood with an implant that allows them to recover hearing capacity. “In our setting, the majority of these patients have a cochlear implant and this excludes the possibility of receiving gene therapy. He has to have a very rare mutation and still have some cells functioning inside the cochlea to make him a possible candidate,” says the otorhinolaryngologist.
He met the characteristics and, with the favor of the family and the medical endorsement, he embarked on the clinical trial to validate a new gene therapy that aimed to restore the little boy’s hearing. The technique consists of introducing into the hair cells, through a non-pathogenic virus (it is not harmful) that works as a “Trojan horse”, Haag exemplifies, the correct instructions to solve the fault with which the boy was born. “There is defective genetic information that causes this protein to not be produced correctly. Well, what is done is a genetic transfer: correct genetic information is taken that reaches the nuclei of the cells and this defect in the production of this protein is repaired, so that from there it can be produced correctly. And with this he can recover his hearing,” says his doctor.
Aissam traveled with his father and Haag to the Children’s Hospital of Philadelphia where he would undergo the new therapy. The intervention, which was performed only on the right ear, lasted a couple of hours, although the boy stayed in the city for three months to undergo exhaustive follow-up.
According to the family, the little boy began to perceive sounds early. Like the noises that came out of the cell phone when the father made the first video call to the family in Spain. His mother says that, in one of the first impressions upon perceiving sounds, the child, “in a gesture that means thanking God,” joined his hands drawing a kind of bowl and expressed with gestures: “Now I am hearing much better than before!”.
One month after the intervention, in the first audiometry and medical studies, the success of the intervention was evident. “This first trial was, above all, to look at patient safety, the functioning of all the application devices, which is why we start with low doses. It was not known if this dose was already sufficient or if it had to be increased. It’s been a bit experimental. The surprise has been that it has worked very well, he has recovered 80% of his hearing in a short time, in less than a month: in the low frequencies he has a slight loss, but in the high frequencies he has normal hearing, although 20 decibels. He has gone from profound deafness to almost normal hearing. It is a very big change. Before he didn’t hear anything and now, when you call him he turns around, he hears how a car sounds or when they cut his hair, he says that these are things that he had never felt before,” explains Haag.
Language difficulties
The child already makes sounds and has even learned to say his name, but doctors see it as unlikely that he will be able to develop language. Gene therapy has allowed him to leave that world silently, but he is late in giving him back the ability to speak. “Everything that is dealt with after these first three years of life and disconnection between ear and brain, the results get much worse because the brain is busy with other things and the auditory pathways no longer develop in the same way,” Haag laments. . It also influences how much one works and is stimulated from a young age, because there are deaf pediatric patients “who can be articulate and talk,” he adds. But this is not the case with Aissam. “If this is not done, then there is no language or understanding. And now he will have the auditory input, but if the connection between the cochlea and the brain is not worked on and he does not have on the hard drive, in the cortex of the brain, a space dedicated to hearing and integrated there, he will improve what he hears or understand, but it will not be enough. Although he will notice improvement,” explains the doctor.
You already notice it, in fact. The boy and everyone around him. Haag himself insists that he is “a very willing, very nice child” and, “although he has not been worked on, he has not received hearing rehabilitation or been taught,” he will learn. “Now he is schooled, he has sign language and can also rely on his auditory sensations. And surely, although neuroplasticity is important in the first years of life, he, throughout his life, will continue to learn and interpret what he hears. Aissam plays soccer, goes to the pool and loves school, a center adapted for children with special educational needs in Santa Coloma de Gramenet. The mother is forceful: “he is very happy.”
Aissam is the beginning of a therapeutic revolution that, if consolidated, can change the care paradigm in this type of patient or in others with deafness due to more prevalent genetic errors. In their same study there is one more child treated in Taiwan and two more to be treated in the United States. Another study, by Chinese researchers, has also shown results with a similar technique. The scientists’ idea, however, is to bring this gene therapy to younger ages so as not to lose that window of opportunity and language learning in the first years of life and so that the impact is as minor as possible.
Haag is enthusiastic about the potential of these treatments, but comes down to earth and calls for caution: “It’s a start with a gene in only one ear. You have to adjust expectations,” he warns. He remains to know the long-term impact or when and how it is best to apply this technique, for example. The doctor asks, above all, not to give up other treatments, such as the cochlear implant, while waiting for a gene therapy like this one that, for now, is only experimental: “The global expectations are good and very interesting, but it is not here still”, agrees.
You can follow EL PAÍS Health and Wellbeing in Facebook, x and instagram.